The MERCURY trials: evidence for Roclanda for the treatment of uncontrolled IOP
Roclanda has the first innovative mechanism of action in topical treatment for primary open angle-glaucoma (POAG) and ocular hypertension (OHT) available in Europe for 25 years4
Roclanda is indicated for the reduction of elevated intraocular pressure (IOP) in adult patients with POAG or OHT for whom monotherapy with a prostaglandin or netarsudil provides insufficient reduction.1
Roclanda was studied in the MERCURY trials 2
MERCURY-1 and -2 were double-masked, randomised, multicentre, active-controlled, parallel-group phase III studies that compared Roclanda (n=483) with netarsudil 0.02% (n=499) or latanoprost 0.0005% (n=486) in adult patients with bilaterial POAG or OHT.3
-
MERCURY-1 and -2 study design3
Primary endpoint Ocular hypertensive efficacy of Roclanda compared with each of its active components (netarsudil and latanoprost) at Week 2, Week 6 and Month 3 at three time points (08:00, 10:00 and 16:00) Secondary endpoints - Mean diurnal intraocular pressure (IOP) at each post-treatment visit
- Proportion of patients achieving prespecified levels for mean diurnal IOP
- Mean change in diurnal IOP at each post-treatment visit
- Ocular and systemic adverse events over 12 months (MERCURY-1) or 3 months (MERCURY-2)
- Safety
Prescribing information and adverse event reporting
Reference: 3. Asrani S, Bacharach J et al. Adv Ther 2020;37:1620-1631
In MERCURY-1 and -2, Roclanda showed superior ocular hypertensive efficacy to netarsudil and latanoprost monotherapy at 2 weeks, 6 weeks and 3 months (primary endpoint; p<0.0001 vs netarsudil and latanoprost for all time points).3
MERCURY-3 study
Roclanda demonstrated comparable IOP lowering to bimatoprost/ timolol2
MERCURY-3 was a prospective, double-masked, randomised, multicentre, active‑controlled, parallel‑group, non‑inferiority study that compared Roclanda (n=218) with bimatoprost 0.03%/ timolol 0.5% (n=212) over 6 months in adult patients with POAG or OHT with insufficient IOP control.2
-
MERCURY-3 study design2
Primary endpoint Ocular hypertensive efficacy of Roclanda relative to Bim/Tim at Week 2, Week 6 and Month 3 at three time points (08:00, 10:00 and 16:00) Secondary endpoints - Intraocular pressure (IOP) at 10:00 at Months 4, 5 and 6
- Mean diurnal IOP
- Mean change from baseline in diurnal IOP
- Mean percent change from baseline in diurnal IOP
- Safety
Bim/Tim: bimatoprost 0.03% and timolol 0.5%
The primary efficacy outcome was non-inferiority in mean IOP reduction between treatments (95% CI ≤1.5 mmHg at all time points and ≤1.0 mmHg at ≥five of nine timepoints) through to Month 3. Roclanda showed clinical non-inferiority relative to bimatoprost/ timolol from Week 2 through to Month 3:2
- ≤1.5 mmHg at all time points
- ≤1.0 mmHg at six out of nine time points
CI: confidence interval
Baseline: 25.10 mmHg
*Unmedicated baseline.2
Safety profile of Roclanda
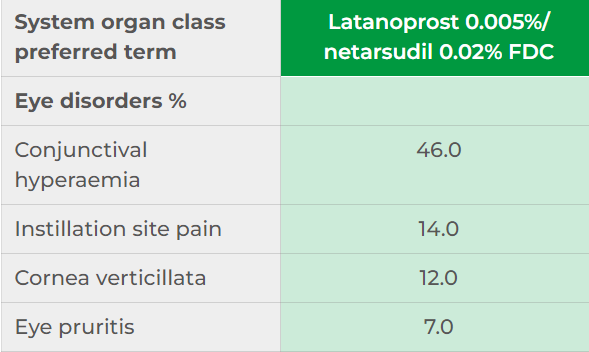
In the MERCURY-1, -2 and -3 trials no serious treatment-related adverse events (TEAEs) were reported with Roclanda.2,3 In the MERCURY‑3 trial more patients discontinued treatment due to ocular TEAEs in the Roclanda group (20.2%) than the bimatoprost/ timolol group (1.9%).2 In MERCURY‑1 and -2, the discontinuation rates over 3 months were higher in Roclanda (8.7%) than netarsudil (7.6%) and latanoprost (1.0%).1
The majority of adverse reactions reported in clinical studies using Roclanda were ocular and mild‑to‑moderate in severity.1 Common ocular events listed in the Summary of Product Characteristics:1
Refer to the Roclanda Summary of Characteristics formore information.