
Roclanda, with netarsudil, is the first innovation in the medical management of glaucoma for 25 years
About
Roclanda is a fixed-dose combination of latanoprost and netarsudil, a ROCK inhibitor1,2
Roclanda is indicated for the reduction of IOP in adult patients with primary open-angle glaucoma or ocular hypertension for whom monotherapy with a prostaglandin or netarsudil provides insufficient IOP reduction.1
Roclanda is a fixed-dose combination of latanoprost and netarsudil that targets trabecular meshwork (TM) dysfunction without losing sight of IOP control.1,3-5
Click on the diagram below to view the Roclanda® MOA
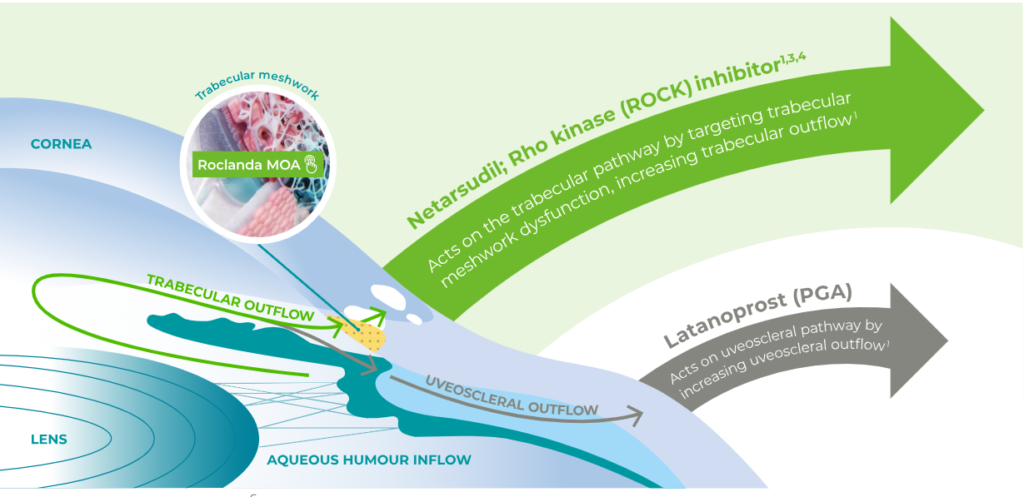
Adapted from Goel M et al, 20106
PGA: prostaglandin analogue
Roclanda targets trabecular meshwork dysfunction lower IOP

*Versus baseline.
See the evidence behind treatment with Roclanda >
Learn more about the trabecular meshwork >
Request Further Information
References
- Roclanda Summary of Product Characteristics
- Schehlein E M, Robin A L. Rho-associated kinase inhibitors: evolving strategies in glaucoma treatment. Drugs 2019;79(10):1031-1036
- Al-Humimat G, Marashdeh I et al. Investigational rho kinase inhibitors for the treatment of glaucoma. J Exp Pharmacol 2021;Volume 13:197-212
- Moshirfar M, Parker L et al. Use of Rho kinase inhibitors in ophthalmology: a review of the literature. Med Hypothesis Discov Innov Ophthalmol 2018;7(3):101-111
- Buffault J, Brignole-Baudouin F et al. The dual effect of Rho-kinase inhibition on trabecular meshwork cells cytoskeleton and extracellular matrix in an in vitro model of glaucoma. J Clin Med 2022;11(4):1001
- Goel M, Picciani R G et al. Aqueous humor dynamics: a review. Open Ophthalmol J 2010;4:52-59
- Buffault J, Labbé A et al. The trabecular meshwork: structure, function and clinical implications. A review of the literature. J Fr Ophtalmol 2020;43(7):e217-e230
- Ahmad S S, Zia-ur-Rahman S et al. Pharmacologic trabeculectomy – modulation of aqueous humor outflow through the trabecular meshwork. US Ophthalmic Rev 2015;8(1):46–51
- Rao P V, Pattabiraman P P, Kopczynski C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: bench to bedside research. Exp Eye Res 2017;158:23-32
- Sturdivant J M, Royalty S M et al. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorganic Med Chem Lett 2016;26(10):2475-2480
- Ren R, Li G et al. Netarsudil increases outflow facility in human eyes through multiple mechanisms. Investig Ophthalmol Vis Sci 2016;57(14):6197-6209
- Kazemi A, McLaren J W et al. The effects of netarsudil ophthalmic solution on aqueous humor dynamics in a randomized study in humans. J Ocul Pharmacol Ther 2018;34(5):380-386
- Sit A J, Gupta D et al. Netarsudil improves trabecular outflow facility in patients with primary open angle glaucoma or ocular hypertension: a phase 2 study. Am J Ophthalmol 2021;226:262-269
- Stamer W D, Clark A F. The many faces of the trabecular meshwork cell. Exp Eye Res 2017;158:112-123